Lab Etiquette (ENG): Difference between revisions
No edit summary |
|||
| (3 intermediate revisions by the same user not shown) | |||
| Line 56: | Line 56: | ||
* Cell transfection: "HEKcell-transfection-Protocol-JWK.pdf" | * Cell transfection: "HEKcell-transfection-Protocol-JWK.pdf" | ||
* In vitro Kinase Assay: "Erik" | * In vitro Kinase Assay: "Erik" | ||
* Immunoprecipitation | * [[Immunoprecipitation]] (MHE) | ||
* Protein expression and purification | * [[Protein expression and purification]] (SBA) | ||
* Lentivirus production: "Marlene" | * Lentivirus production: "Marlene" | ||
* Bacterial growth curve: "Ann-Kathrin" | * Bacterial growth curve: "Ann-Kathrin" | ||
| Line 110: | Line 110: | ||
'''Thesis:''' for your thesis: for '''every''' figure in your thesis create a subfolder named with the Figure-Number and include in each subfolder both: the final figure as pdf- and illustrator-file and the '''used raw data''' (subdivided in panels A, B, etc.). The name of the raw data file should remain the same as it is found in your DATA-folder and lab book) | '''Thesis:''' for your thesis: for '''every''' figure in your thesis create a subfolder named with the Figure-Number and include in each subfolder both: the final figure as pdf- and illustrator-file and the '''used raw data''' (subdivided in panels A, B, etc.). The name of the raw data file should remain the same as it is found in your DATA-folder and lab book) | ||
[[File:Labetiquette fileserver.jpg|thumb|center|696x696px|'''Fig. 1:''' Example of the user folder structure on the Fileserver_LSH_Data of user UE_Universal_Example.]] | |||
== Laboratory Workspace == | == Laboratory Workspace == | ||
Latest revision as of 10:19, 16 June 2025
Diese Seite ist auch auf Deutsch 🇩🇪 verfügbar.
Welcome to the lab – think together, act together!
A well-functioning lab doesn’t rely solely on rules but on mutual awareness and shared responsibility. Especially for newcomers, it’s better to ask one question too many than to do the wrong thing silently. If you notice that something is running out – let people know. If you change something, inform others. That’s how we keep things smooth, safe, and effective.
Laboratory Manual - Cell Biology Research Group
Laboratory Access
The ML laboratory building is open on weekdays from 6:30 AM to 8:30 PM. Access to laboratory rooms is obtained using RFID transponders, which also allow entry to the laboratory building outside the above opening hours. Master's students and doctoral candidates receive a personal transponder through key distribution (form available from Petra Schnurr) and must return the transponder after completing their time as employees. For bachelor's students and research assistants, a loan transponder is available in exceptional cases after training for necessary work outside opening hours. The loan transponder is located in the key cabinet at the general computer workstation. Removal of the loan transponder must be noted in the list with date and initials.
Working Hours
Non-scientific staff and research assistants are required to record their working time. The online website of the personnel administration is available for this purpose:
https://personal.uni-konstanz.de/qisserver/rds?state=user&type=0&topitem=
Research assistants use timesheets for time recording (available from Petra Schnurr).
Scientific staff should be present during core working hours 9:00 AM – 12:00 PM and 2:00 PM – 4:00 PM. Absence must be discussed with Christof and noted in the wall calendar in the entrance area of the office row.
Illness
In case of illness, Christof and the secretariat should be notified as soon as possible by telephone and/or email so that coverage, etc. can be organized. For prolonged illness, a medical certificate/medical attestation must be sent to petra.schnurr@uni-konstanz.de on the third day of absence.
Vacation
Employees should apply for vacation as early as possible (at least 1 week in advance) through the online time recording system:
https://personal.uni-konstanz.de/qisserver/rds?state=user&type=0&topitem=
The possibility for long-term vacation (> 2 weeks) is project-dependent and should be discussed with Christof at least 2 months in advance. During the second half of the winter semester (early January – end of February), vacation during the VTK is only possible in exceptional cases.
Before taking vacation, the culture/storage of important clones/cell lines/samples must always be ensured and coverage for lab duties must be organized. Bachelor's/Master's students should discuss multi-day absences with their supervisor and Christof.
Seminars/Project Meetings
Monday mornings (8:45 AM) there is a general lab meeting. This is followed by project presentations by individual staff members, which are announced on the notice board in the entrance area.
Wednesday afternoons from 3:15 PM – approximately 5:00 PM our journal club takes place. The publication being discussed is loaded onto the file server or sent as a PDF file to everyone the week before. Every two weeks in this seminar, we discuss current new data from all staff members.
Participation in both seminars is mandatory; the seminar schedule is usually set three to six months in advance.
Tuesday afternoons and Thursday lunchtimes during the lecture period, the seminars of the graduate school KoRS-CB as well as the departmental seminar of the Biology Department take place. Speakers/topics are announced by posting. Participation is optional, but when possible, one should not miss interesting presentations.
Specific project meetings take place by arrangement with Christof. Lab books should be brought to project meetings as a basis for discussion.
Name Abbreviations/Labels
Each employee uses a distinctive name abbreviation with which all samples, solutions, etc. must be labeled. Name abbreviations are assigned by the secretariat and are stored in a list on the file server:
Fileserver_LSH/Templates/Lab-Directories/Name-Abbreviations_AG Hauck_2025.doc
Lab Book
Each employee maintains a lab book, which is issued by the secretariat and in which all work is documented in chronological order. Templates for documenting various experiments are stored as PDF files on the file server:
Fileserver_LSH/Templates/Lab-Documentation/
- Gentamicin Assay: "Steffi"
- PCR: "PCR-based-LIC-cloning-protocol-CRH.pdf"
- Cell transfection: "HEKcell-transfection-Protocol-JWK.pdf"
- In vitro Kinase Assay: "Erik"
- Immunoprecipitation (MHE)
- Protein expression and purification (SBA)
- Lentivirus production: "Marlene"
- Bacterial growth curve: "Ann-Kathrin"
The pages of the lab book are numbered consecutively and a table of contents is created on the first two pages.
The lab book belongs to the Cell Biology Department and remains the property of the department after departure. No pages may be removed from the lab book. Original printouts, gel copies, X-ray films, etc. are labeled and attached to the lab book by being stapled or glued to the corresponding experiments. Electronic original data is stored on the file server in a separate user directory (see Data Archiving chapter).
For most experiments, there are already lab protocols that are described in detail in our VTK script and can be referenced in the lab book. The VTK script can be found on the file server:
Fileserver_LSH/Templates/Lab-Documentation/Script-Advanced-Course-2025.doc
The reagents and solutions used are also described either in the recipe file: Fileserver_LSH/Recipes/Recipes-2025.doc or can be found in the chemical list:
Fileserver_LSH/Chemical-List/Chemical-List 2025 Group Hauck.xls
Only when deviating from existing protocols are special notes in the lab book necessary.
For microscopic work, flow cytometric analyses, or similar work with results in electronic form, a sample protocol is prepared (see Fileserver_LSH/Templates/Lab-Documentation/Protocol-Microscope-FACS.doc") and attached to the experiment.
During project meetings, the lab book serves as the basis for discussing results and should therefore definitely be brought along.
Data Archiving
Standard experiments should be performed according to the lab protocols listed above; deviations should be recorded in the lab book. The raw data obtained during experiments (e.g., microscopy images, gel images, FACS data, VarioScan data) as well as derived figures created from them (graphics, processed microscopy images, etc.) should be stored on the data file server "Fileserver_LSH_Data/00_User/" in a separate user folder. Only there is automatic (daily) backup performed, so no data can be lost.
Each electronic data file contains the date, name abbreviation, and experiment in the name (The raw data files are named according to the following scheme):
Year-Month-Day_Name-Abbreviation_Experiment / year-month-day_initials_experiment
e.g. /e.g.: 2025-01-01_CRH_Adhesion assay
- the digital version/raw files should be on the file server with the date that can also be found in the lab book
- a printed version of the evaluated data should be attached in the lab book
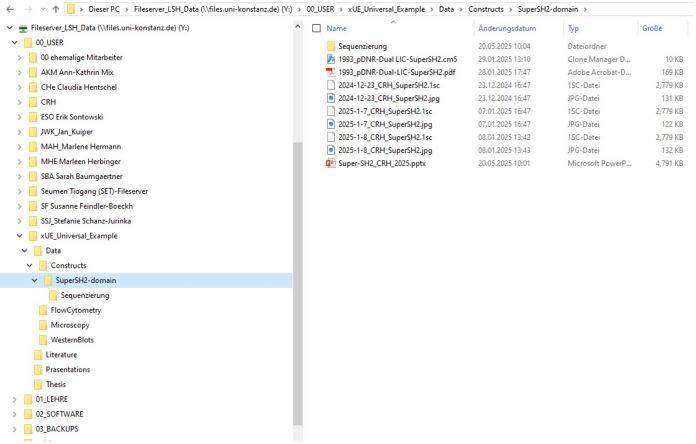
For later use in publications it is important that we all stick to the same folder structure and naming of files. An example of such a user folder is given on the Fileserver and depicted on the next page:
Fileserver_LSH_Data/00_User/xUE_Universal_Example/
Data
- Constructs
- SuperSH2 agarose gels, sequencing results, plasmid map, etc.
- Western Blots
- Immunofluorescence staining
- Flow Cytometry
- ELISA/SEAP-Assays
- ...
Literature
Presentations
Thesis: for your thesis: for every figure in your thesis create a subfolder named with the Figure-Number and include in each subfolder both: the final figure as pdf- and illustrator-file and the used raw data (subdivided in panels A, B, etc.). The name of the raw data file should remain the same as it is found in your DATA-folder and lab book)
Laboratory Workspace
In the laboratories, there are general laboratory workspaces (e.g., in the two cell culture labs, in the infection room, or the area with protein gel electrophoresis) as well as private workspaces that are assigned. Work at private workspaces of others should only occur after consultation. Everyone is responsible for keeping their workspace, associated shelves, and drawers clean. The work surface is wiped down and disinfected at least once per week; drawers/shelves should be dusted regularly and kept tidy.
Additionally, everyone has a storage space in a refrigerator at 4°C, in a freezer/compartment at -20°C, and can store samples in labeled boxes in the -80°C freezer in room ML. Plastic boxes with lids are available for storage; open stands (especially for Eppendorf tubes) should only be used for short-term storage (1 day). All samples (Eppendorf tubes, agar plates, etc.) must be labeled with abbreviation, content description, and date. All storage locations should be organized/updated regularly (at least once per month). For storage in the -80°C freezer, a data sheet must be filed in the -80°C folder for each box. A template for the data sheet can be found on the file server:
Fileserver_LSH/Templates/Lab-Documentation/-80C Box-Template.doc
Please reduce opening of deep freezers to a minimum (otherwise icing and malfunction). Therefore, think before opening about what should be removed and where it can be found (see data sheet!!!). General laboratory workspaces should be cleaned after each use (e.g., sterile bench, protein gel electrophoresis, fume hood, scales, gel documentation) and all used solutions, equipment, etc. must be put away.
Lab Jobs
Each employee receives a lab job that they perform for the general community. The lab job can include maintaining a general collection, responsibility for a device, or supervision and organization of a laboratory. In addition, the preparation of solutions/buffers etc. for the general community is handled through lab jobs. Those responsible are listed on the notice board at the entrance. Room supervisors also organize regular maintenance/cleaning schedules for general equipment such as sterile benches, incubators, etc.
Closing Service
The evening closing service, which ensures that equipment such as sterile benches, vacuum pumps, gel documentation, or the propane gas line are turned off, rotates weekly among scientific staff and master's students. A list for the evening control round of the closing service hangs on the notice board at the entrance.
Computer Use
There is a computer for general use in the seminar room. Please always enable use for official purposes. Private PCs/notebooks can be connected to the network. A current virus scanner and activation of a firewall are required. Access to the department's network storage in the computing center (Fileserver_LSH and Fileserver_LSH_Data) is set up by Christof. Various software programs are available on Fileserver_LSH_Data/02_SOFTWARE, including Adobe Photoshop CS4, Adobe Acrobat Pro9, Adobe Illustrator CS4, MS Office 2016, Clone Manager 9, Endnote X9, SigmaStat 4.0, Leica LAS, Biorad QuantityOne, VarioScan SkanIt. To ensure smooth data exchange within the lab, the above-mentioned versions of these programs must be used for all official purposes, or the data must be saved in a format compatible with these program versions.
Everyone's Cooperation is Required
Each employee can contribute with little effort to minimizing additional work in the laboratory for everyone. Thus, everyone should keep consumption/waste/cleanup effort as low as possible through forward-thinking planning and also help with waste disposal. Please do not fill autoclave bags until "overflowing," but close them in time, deposit them in the tray, and put a new autoclave bag in the corresponding waste container.
Everyone is also responsible for filling boxes with pipette tips and refilling Eppendorf tubes. Autoclave materials are collected in the plastic box and autoclaved by Claudia/Susanne for everyone.
Proper behavior also includes conduct and cleanliness in the tea kitchen and social room. If I have never loaded or unloaded the dishwasher or refilled the coffee machine during the course of one month, then I have very likely used clean dishes at the expense of others.
Chemicals/Solutions
Many solutions are prepared as lab jobs and either kept accessible to everyone as stock solutions in designated places or provided as aliquoted solutions, where one takes an aliquot to their own workspace as needed and stores it there for further use. Additional solutions for personal use should be prepared yourself (please estimate the required amounts in advance and do not prepare liter-wise solutions when you only need 10 ml for two experiments in total) and correctly labeled (abbreviation, date, content: designation and concentration; hazard symbol) and stored at your own workspace. Use solutions and aliquots at colleagues' workspaces ONLY after consultation. Stock solutions, aliquoted solutions, and the respective responsible persons are listed on the "Lab Jobs" bulletin board. For preparing solutions, there is a recipe book available as a file on the file server:
Fileserver_LSH/Recipes/Recipes-2025.doc
General Collections
The department maintains various collections that represent an important resource for everyone and must therefore be handled with particular care and responsibility.
Strain Collection: includes all bacterial strains that are filed by numbers. Storage at -70°C in chest on level 5 as well as in room ML6:
- non-pathogenic bacteria (= simultaneously repository of all produced plasmids)
- pathogenic bacteria and mutants (key available from Christof)
The strain collection is also available as an MS Access file. Each newly produced or externally obtained bacterial strain receives a new strain number and is frozen in the strain collection as well as electronically recorded. Number distribution is handled by Christof. Only Susanne and Christof have access to the strain collection.
Plasmid Collection: includes all isolated plasmids from non-pathogenic bacterial strains of the strain collection. Numbering is identical to the strain collection (storage in general refrigerator at 4°C)
Cell Collection: includes stocks of all cell lines and primary cells. Stored in liquid nitrogen in the media kitchen. The cell collection is managed by Susanne.
Virus Collection: includes all lentiviral, adenoviral, AAV particles, and bacteriophages. Stored at -70°C. Access file!
Oligo Collection: includes all oligos ordered for cloning, PCRs, etc. Oligos are stored as 10 mM stock solution in TE buffer at -20°C in the general collection, not in private freezers. Ordering new oligos and filing information is handled by Jan Kuiper.
Antibody Collection: Collection of all primary antibodies (commercial or self-made) and secondary antibodies (enzyme- or fluorescence-conjugated). Data sheets are collected in the "Antibody" folder. Claudia Hentschel organizes the antibody collection.
Protein Collection: All recombinant, purified proteins are recorded here.
Inhibitor Collection: Information on all pharmacological inhibitors and the concentration, solvent, and storage location of stock solutions are filed here. _______________ organizes the inhibitor collection.
Enzyme Collection: includes all commercial and self-made (DpnI) restriction enzymes stored at -20°C. Removal of enzymes from stock containers only with FILTER TIPS!
Enzymes may only be removed from the freezer for the shortest possible time (1-2 minutes) (i.e., directly before use) and are stored during this short time in a paraffin block cooled to -20°C.
Folders with documentation for all collections can be found on the shelves at the general workspace in the office area.
Competent Cells
Aliquots of competent bacteria are stored at -80°C. 200 μl are frozen per aliquot, sufficient for 2 transformations. Various strains are available for different purposes. The standard cloning strain is NovaBlue. When removing, proceed as quickly as possible; warming of aliquots leads to reduced competence; do not remove more aliquots than actually needed.
Enzymes
Polymerases, restriction enzymes, etc. are stored at -20°C in boxes or on paraffin blocks together with the corresponding buffers. These enzymes are used jointly by everyone. Therefore, handle enzyme stock solutions particularly cleanly and avoid contamination (filter tips!!!) as well as warming.
When removing enzymes, transport the Eppendorf tubes with the stock solution exclusively in a paraffin block cooled to -20°C to the workplace and leave them in it for pipetting. Even with paraffin block, remove the enzyme from the -20°C freezer only briefly (< 2 minutes) and return it immediately after pipetting. When opening stock containers, definitely avoid contact of the inner lid surface with skin!!
Orders
When general chemicals or disposables are running low, give notice IN TIME (i.e., before using up the last package or pipetting the last microliter): either write on the board in the lab or tell the orderers. Discuss project-specific chemicals/antibodies etc. or lab utensils/equipment with Christof before ordering.
For oligo orders through Jan, enter the oligo information in the order list on the file server: Fileserver_LSH:\Strain-Collection\Primer\01_PrimerOrdering_Primer-Order
Discuss other online orders with Christof.
Order sequencing using pre-paid labels from LGC or GeneWiz. Please no more than 2 sequencing per new construct. Discuss larger sequencing needs with Christof. Deliveries of reagents should be handed over to the orderer. The orderer checks for completeness (using the delivery slip) and confirms with date and signature. The delivery slip and any accompanying invoices are forwarded to Petra Schnurr in the secretariat. When chemicals, antibodies, enzymes, and other reagents are delivered, the data sheets are dated and filed in the corresponding folders (see "General Collections"). The solvent, concentration, and possibly filled aliquots as well as storage location may be noted on the data sheets.
ALL chemicals, reagents, enzymes, etc. are stored in accessible and known locations.
Laboratory Safety
See Safety Guidelines.