Protein Phosphatase Assay
| Materials | |
|---|---|
| black flat-bottom 384-well plates (Greiner Bio-One, Germany) | |
| phosphatase buffer (50 mM TrisHCl, pH = 8.0 at 25°C, 200 mM NaCl, 20 mM MnCl2, 2 mM TCEP, 0.01% Tween). TCEP and Mn2+ are added freshly; 1L bottle is sterile filtered and stored at 4°C. | |
| 4-Methylumbelliferyl phosphate (4-MUP) (Sigma Aldrich, Germany) or DiFMUP (ABCR GmbH) is dissolved in DMSO (10 mM stock solution) and stored at -50°C | |
| recombinantly expressed phosphatase (-80°C) | |
| compound aliquots (-80°C) and DMSO for pre-dilution |
Assay Procedure
1.) Prepare buffer with and without Mn2+ in appropriate amount
2.) Pre-dilute substances in DMSO in a 96 deep-well plate (can be frozen at - 80°C)
| Endkonzentration im Assay [µM] | Total- Faktor | Verdünnung in DMSO [µM] | |||||||
| 10 | X1000 | 10000 | → | 0 | µl DMSO | + | 5 | µl aus | 10mM |
| 3,3 | X1000 | 3333 | → | 6 | µl DMSO | + | 3 | µl aus | 10 |
| 1 | X1000 | 1000 | → | 7 | µl DMSO | + | 3 | µl aus | 3333 |
| 0,3 | X1000 | 333 | → | 6 | µl DMSO | + | 3 | µl aus | 1000 |
| 0,1 | X1000 | 100 | → | 7 | µl DMSO | + | 3 | µl aus | 333 |
| 0,03 | X1000 | 33 | → | 6 | µl DMSO | + | 3 | µl aus | 100 |
| 0,01 | X1000 | 3 | → | 7 | µl DMSO | + | 3 | µl aus | 33 |
| 0 | X1000 | 0 | → | 7 | µl DMSO |
3.) Pre-dilute DMSO dilution row again in assay buffer (125 µl buffer and 0.5 µl DMSO dilution) in another 96 deep-well plate to result in 1:250 dilution (in the assay again 1:4 dilution is done to result in a total dilution of 1:1000).
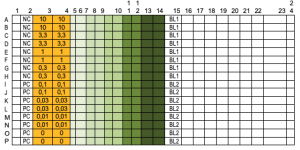
4.) Freshly prepare phosphatase enzyme solution using appropriate amounts of phosphatase. PC = phosphatase with Mn2+ buffer (60µl per well); NC = phosphatase w/o Mn2+ (60µl per well); samples = phosphatase with Mn2+-buffer (40µl per well). Freshly prepare DiFMUP/4-MUP solution by adding appropriate amounts to assay buffer w/o Mn2+.
5.) Add 80µl of one buffer in the first and the last column of a 384-well plate (not measured because of effects in the reader); add 60 µl/well (controls) or 40 µl/well (samples) of phosphatase solution for controls and samples; add 60µl/well of buffer with and w/o Mn2+ for background detection during the assay into wells (Blanks = BL1 and BL2).
6.) Add 20 µl of different compound solutions (10µM - 0µM) to the sample wells, mix and pre-incubate for 10 min at 35°C under constant shaking (5 Hz).
7.) For starting the reaction add 20 µl of the substrate solution to all wells yielding a final concentration of 100 µM DiFMUP/4-MUP.
8.) Fluorescence intensities of the hydrolyzed 4-Methylumbelliferone (4-MU) or DiFMU (excitation 360nm/emission 448nm) are monitored by a microplate reader (Varioscan, Thermo Fisher Scientific) in a kinetic mode every 5 min over a 60 min period at 35°C.
9.) For calculation of final values normalize to auto-hydrolysis (blanks) and autofluorescence of compounds (first time-point). You can draw curves with GraphPadPrism and align IC50/EC50 curves or use end-point values etc.