Protein expression and purification
Expression of HisSUMO-hPOPX2 (SAK) – Part 1/2
- Bacteria Sample: #570 BL21 DE3 pRos. pET24a His-SUMO_hPopx2_fulllength
- One day prior, aprox. 15:00 : inoculate 50 mL of antibiotics containing media with 3 colonies and incubate o/n at 37°C
- 7 mL each of overnight culture were used to inoculate 3 x 333 mL of antibiotics (Kan, cam) containing LB-medium in a 1 L Erlenmeyer flask until OD = 0.7
- When OD is reached, Erlenmayers were shortly transferred onto ice for temperature reduction
- 0.5 ml is removed (sample before induction), centrifuged for 1 min at 13000 rpm and the pellet is resuspended in 100 μl 2xSDS sample buffer and heated for 5 min at 90°C.
- Bacterial cultures were induced with 0.5 mM IPTG and subsequently incubated at 20°C upon shaking (160 rpm) for 4 h
- 0.5 ml is removed (sample after induction), centrifuged for 1 min at 13000 rpm and the pellet is resuspended in 100 μl 2xSDS sample buffer and heated for 5 min at 90°C.
- Remaining cells are centrifuged for 30 min at RT with 4700 rpm (be careful to only use the old centrifugation flasks with the old centrifuge under the incubator and do not use those with the big centrifuge in front of ZK)
- Carefully remove supernatant and resuspend pellet in remaining approx. 15 mL supernatant
- Transfer these 15 mL of resuspension into a 50 mL Falcon
- Centrifuge at 4700 rpm for 15 min
- Aspirate supernatant and freeze pellet at -80°C
PPM1F Expression in Bacteria - Recombinant hPOPX2 (PPM1F) purification (conducted by SF) – Part 2/2
Lysis of bacteria:
- Thaw bacterial pellet at 4°C
- Resuspend in 15 mL Buffer A with protease inhibitors (1 µM PMSF, 10 µg/mL aprotinin, 10 µg/mL pefablock, 5 µg/mL Leupeptin)
a. BUFFER A: 50 mM NaPhosphat, 1 M NaCl pH 8.0
- Sonificate: 3 x 2 min 4°C with approx.. 5 min break inbetween
- Centrifuge for 30 min at 18000 rpm
- Filtrate supernatant through a 0.2 µm pore size filter and apply onto column
Purification via column:
- COLUMN: HisTrapFFcrude 1 mL (Pharmacia), equilibrate with 20 mL 50 mM NaPhosphate buffer, 1 M NaCl, pH 8.0 1 mL/min
- Apply supernatant post centrifugation with 0.5 mL/min
- Wash 60 min with buffer A + 10 mM Imidazol 1 mL/min, 4°C
- Elution: Gradient 0-50% Buffer B for 40 min; 20 min 50% Buffer B; collect fractions with 3 min/fraction ~ 3 mL per fraction
a. BUFFER B: 50 mM NaPhosphat, 0.5 M NaCl, 0.5 M Imidazol pH 8.0
- Wash column for 20 min with 100% buffer B
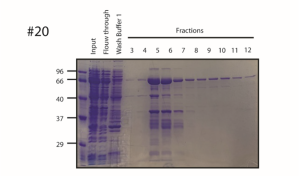
Gel-Electrophoresis post purification of recombinant hPOPX2 (PPM1F)– Gel #20
- Purification of recombinant PPM1F via HisSUMO tag conducted by SF
- Mix samples with 2x SDS loading buffer
- Load samples onto 12.5% Acrylamide gel
- Perform SDS-Page at 80V/120V
- Coomassie stain gel after short heating in the microwave for 30 min at rt
- Destain until bands are visible
PPM1F Expression in Bacteria – Dialysis (conductec by SF)
- Dialysis in 1.5 L o/n against 20 mM Tris pH8, 150 mM NaCl, 1.5 mM MgCl2 (no glycerin!)
- Dialysis in 1.5 L for 4 h against 20 mM Tris pH8, 150 mM NaCl, 1.5 mM MgCl2 (no glycerin!)
- Dialysis in 1.5 L for 4 h 30 min against 20 mM Tris pH8, 150 mM NaCl, 1.5 mM MgCl2 (no glycerin!)
- Observation: some precipitates form, centrifuge 3000 rpm 10 min at 4°C
Quantification Gel for recombinant hPOPX2 (PPM1F)– Gel #21
- Dialysis performed by SF
- Mix samples with 2x SDS loading buffer
- Load 2.5 or 5 µL of sample onto a 12.5% Acrylamide Gel
- Perform SDS-Page at 80V/120V
- Coomassie stain gel after short heating in the microwave for 30 min at rt
- Destain until bands are visible
→ Impurities mainly in the fallout fraction (“Pellet”); Supernatant contains approx.. 400 ng/µL HisSUMO-POPX2
Ulp1 treatment for His-SUMO tag cleavage of His-SUMO hPOPX2 – Part 1/2
- Incubate 5 mL of purified His-SUMO hPOPX2 (conc. 400 ng/µL) with 5 µL Ulp1 (conc. 2 mg/mL) → 1 µg of Ulp1 per 200 µg of sample
Purification post Ulp1 treatment for His-SUMO tag cleavage of His-SUMO hPOPX2 – Gel #24 – Part 2/2
- Prime column with wash buffer (50 mM Sodium Phosphate buffer pH 8 with 1 M NaCl) Install tubing of column in the pump (the tube is inserted from the bottom side in)
- The column is hung upside down
- Pump water at 0.5 ml/min for about 10 min, fill tubing first, then connect to column (?)
- Remove the tube from the end of the column and insert column the right way round
- Prime column with wash buffer (50 mM Sodium Phosphate buffer pH 8 with 1 M NaCl) 0.5 mL/min, 15-20 mL
- Prepare 6 Schnappdeckelröhrchen
- Prepare 3 mL sample by addition of 1:50 elution buffer (50 Mm Sodium phosphate buffer pH 8, 0.5 M NaCl and 0.5 M imidazole) and 1:10 of 5 M NaCl-stock solution)
- Pump sample onto column, collect flow through (DF, Durchfluss) → hPOPX2 should be eluted here
- Start with Elutionbuffer 1 (50 mM Sodium phosphate buffer pH 8, 1 M NaCl, 10 mM Imidazole): per Schnappdeckelröhrchen pump 0.5 mL/min for 6 min → 3 mL/fraction collected. Collect three fractions with Elutionbuffer 1
- Switch to Elutionbuffer 2 (50 mM Sodium phosphate buffer pH 8, 1 M NaCl, 0.5M Imidazole): per Schnappdeckelröhrchen pump 0.5 mL/min for 6 min → 3 mL/fraction collected. Collect three fractions with Elutionbuffer 2
- Wash column with elution buffer 2 for 15 min at 0.5 mL/min
- Mix 20 µL of sample with 20 µL 2x SDS, boil 5 min at 95°C and apply 20 µL to 12.5% SDS-Page
- Run SDS-Page at 80V /120 V
- Stain gel with coomassie for 45 min at rt after short warming in microwave
- Destain until bands are visible
Cleaning of Column after use:
- wash 10 min 1 mL/min with Elutionbuffer 2 (10 mL)
- wash 20 min 1 mL/min with Wash buffer (20 mL)
- wash 20 mL 1 mL/min with H2O (degassed)
- Wash 20 mL 20% EtOH
Quantification Gel for recombinant hPOPX2 (PPM1F) after Ulp1 treatment and dialysis – Gel #26
- Mix samples with 2x SDS loading buffer
- Boil samples 95°C for 5 min
- Load onto a 12.5% acrylamide gel
- Run at 80 V/120 V
- Coomassie stain after short heating in microwave for 40 min
- Destain until bands become visible
Concentration Determination of purified recombinant hPOPX2 (PPM1F)
- Determine Protein Conc. Via 660 nm Pierce Protein Assay Kit (ThermoScientific)
o Pipette 10 µL of BSA standard in PBS or 10 µL of blank or 10 µL of sample in triplicates into a 96 well plate
o Add 150 µL Pierce 660nm Protein Assay Reagent (storage: rt, JWK)
o Cover plate and mix on a plate shaker at medium speed for 1 minute. Incubate at room temperature for 5 minutes.
o Measure absorbance at 660 nm
o Perform standard curve and calculate the concentration of the protein sample
→ Sample Concentration: 131,1027 ng/µL in 9 mL dialysis buffer